ANDA: What It Is, Why It Matters for Generic Drugs
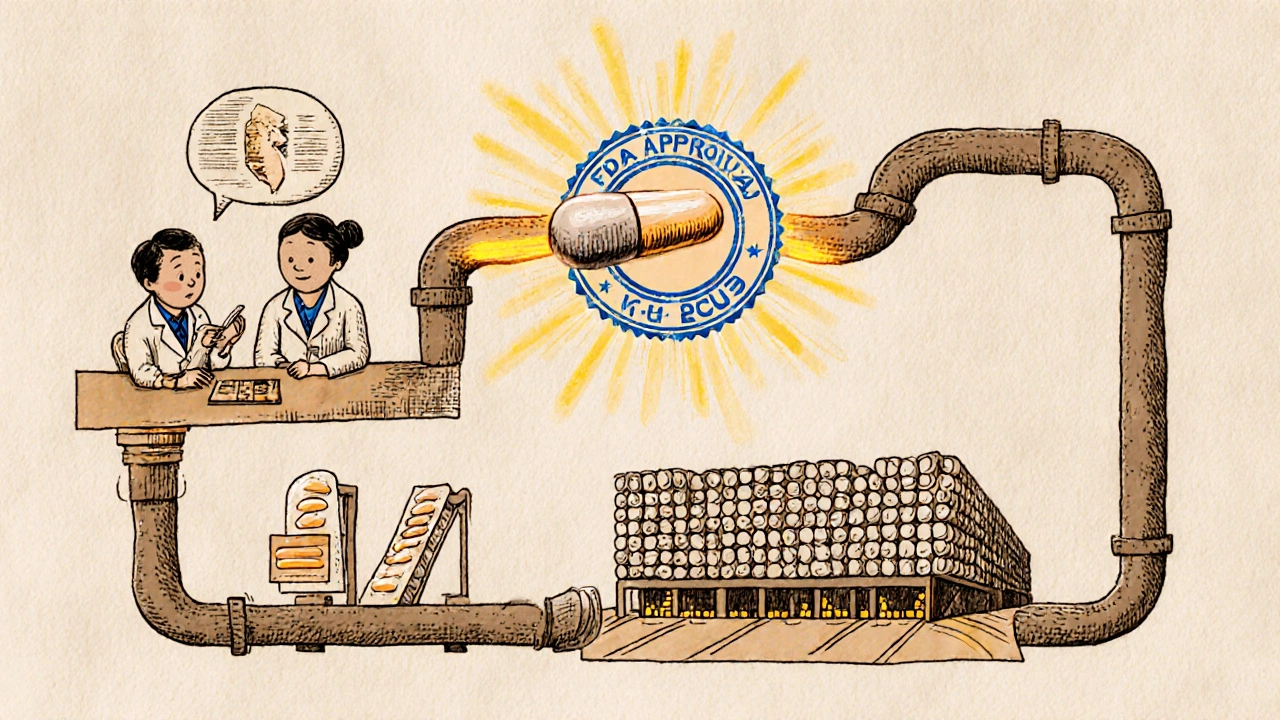
When you pick up a generic pill at the pharmacy, chances are it got there through an ANDA, Abbreviated New Drug Application, the FDA’s official process for approving generic versions of brand-name medications. Also known as Abbreviated New Drug Application, it’s the backbone of affordable medicine in the U.S. Without ANDA, most generic drugs wouldn’t be legal to sell—meaning higher prices for everyone.
Every ANDA must prove the generic version is bioequivalent to the brand-name drug. That means it delivers the same amount of active ingredient into your bloodstream at the same rate. The FDA doesn’t require new clinical trials for every generic—instead, they rely on in vitro, lab-based tests like dissolution studies and sometimes in vivo, human absorption studies to confirm this. This is why you see so many posts here comparing generic versions of drugs like sildenafil, finasteride, or cefdinir—they’re all products of the ANDA system.
ANDA isn’t just about science. It’s about access. It’s what lets a 30-day supply of lisinopril cost $4 instead of $300. It’s why insurers push for generics—they know the FDA has already verified safety and effectiveness. But not all ANDAs are created equal. Some get approved quickly with simple data. Others face delays if the manufacturer can’t prove consistent quality. That’s why some generics work better for you than others, even if they’re labeled the same.
Behind every generic you take is a paperwork trail—the ANDA—that includes details on ingredients, manufacturing sites, stability testing, and packaging. The FDA inspects these facilities. If something’s off, they can reject the application or pull the drug from shelves. That’s why you should only buy generics from trusted sources. Sites selling cheap pills without clear labeling or FDA tracking often skip the ANDA process entirely—and that’s dangerous.
You’ll find posts here that dig into how ANDA affects everything from insurance prior authorizations to why some generic inhalers cost more than others. You’ll see how bioequivalence testing shapes choices between brands. You’ll learn how drug manufacturers navigate the system to get their products approved—and how that impacts what’s on your shelf.
Whether you’re a patient wondering why your new generic feels different, a pharmacist explaining coverage rules, or just someone trying to save money on prescriptions—understanding ANDA helps you ask the right questions. Below, you’ll find real-world breakdowns of generic drugs, regulatory hurdles, and what actually happens before that bottle hits your medicine cabinet.
- 14 Comments
Discover how generic drugs go from FDA approval via ANDA to being available at your local pharmacy. Learn about the science, regulation, and supply chain that make affordable medications possible.
