In Vivo Bioequivalence: What It Means for Generic Drugs and Your Health
When you pick up a generic pill, you’re trusting that it does the same thing as the brand-name version—without the higher price. That trust comes from in vivo bioequivalence, a testing method that compares how a drug behaves inside the human body. Also known as human pharmacokinetic study, it’s the gold standard for proving that generics are therapeutically equal. Unlike lab tests that just check chemical composition, in vivo bioequivalence looks at real-world absorption: how fast and how much of the drug enters your bloodstream, and how long it stays active. This isn’t theoretical—it’s required by the FDA before any generic drug hits the shelf.
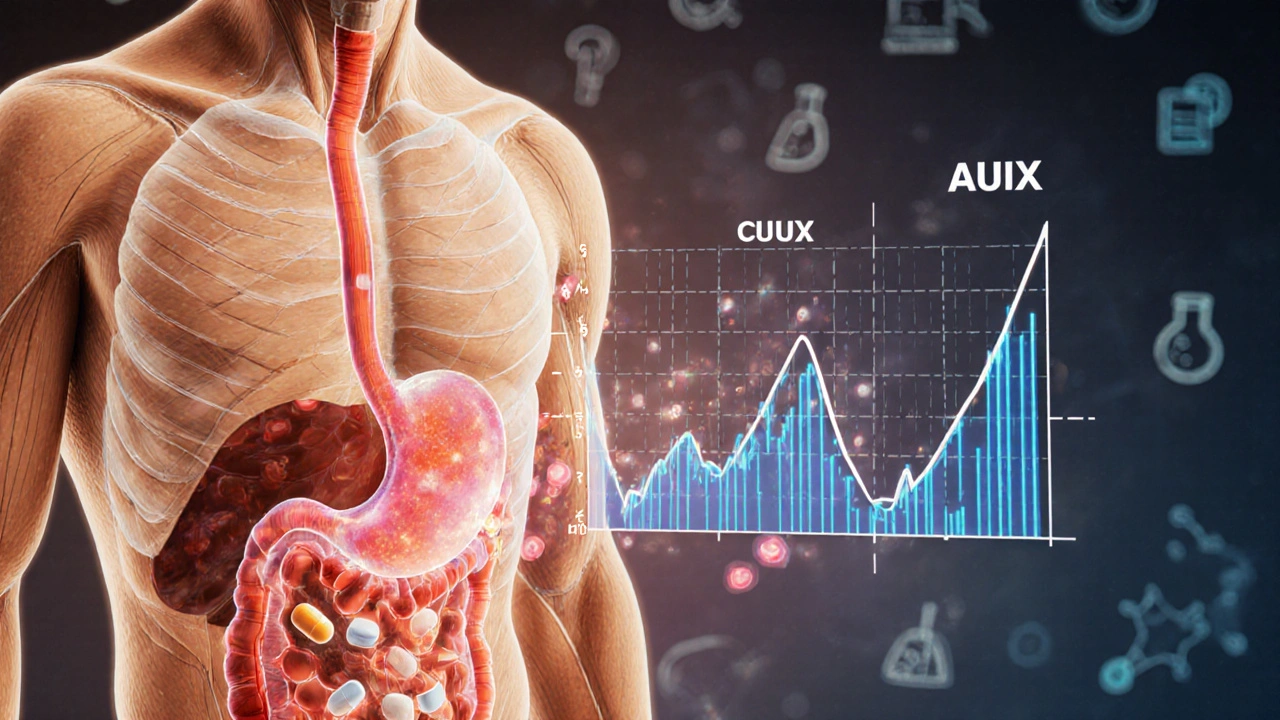
Behind every generic medication you use, there’s a study where healthy volunteers took both the brand and generic versions, then had their blood drawn over hours to map out the drug’s journey. These studies measure key numbers: Cmax, the highest concentration of drug in the blood, and AUC, the total exposure over time. If those numbers fall within 80–125% of the brand drug’s values, regulators approve the generic. This isn’t a loophole—it’s science. And it’s why you can safely switch from brand-name Lipitor to generic atorvastatin without worrying your blood pressure won’t be controlled.
But not all drugs are created equal when it comes to bioequivalence. Some, like warfarin or levothyroxine, have narrow therapeutic windows—tiny differences in absorption can cause real harm. That’s why these drugs get extra scrutiny. Meanwhile, drugs like ibuprofen or metformin have wide margins, so even if absorption varies slightly, the effect stays safe. The posts below dive into how this plays out in real life: how generics make it to pharmacies, why insurers push them, and what happens when a drug’s bioequivalence is questioned. You’ll find real examples—from how Fildena and Finpecia compare to their branded cousins, to why some people still doubt generics despite decades of proven safety. This isn’t just about chemistry. It’s about your health, your wallet, and the quiet system that makes affordable medicine possible.
- 8 Comments
In vivo bioequivalence testing uses human trials to measure drug absorption, while in vitro methods rely on lab tests like dissolution. Learn when each is used for generic drug approval and why regulators are shifting toward in vitro for simpler products.
