Allergy & Autoimmune Risk Calculator
Your Risk Assessment
Enter your details and click "Calculate Risk" to see your risk level.
When you hear someone talk about allergies or an autoimmune condition, you probably picture two completely different health problems. One feels itchy, the other can be life‑changing. Yet growing evidence shows that allergic disorders and autoimmune diseases share more than just a name - they often arise from the same immune misfires. This article unpacks that connection, explains why it matters for patients and clinicians, and offers practical steps you can take today.
Quick Take
- Both allergies and autoimmunity stem from an over‑active immune system that reacts to the wrong target.
- Shared genetic variants (e.g., HLA‑DR, IL‑4R) and environmental triggers (dust, pollutants, diet) increase the odds of having both conditions.
- Common pathways like chronic inflammation, disrupted gut microbiome, and faulty regulatory T cells link the two disease groups.
- Patients with one condition are up to three times more likely to develop the other.
- Integrated management-targeting inflammation, restoring gut health, and monitoring biomarkers-can improve outcomes for both.
What the Immune System Actually Does
At its core, the immune system is a network of cells, proteins, and organs that protects the body from infections and foreign invaders. When a pathogen shows up, white blood cells like T‑cells and B‑cells launch a targeted response, releasing antibodies and cytokines to neutralize the threat.
Two key safety nets keep this response in check:
- Regulatory T cells (Tregs) - they act like traffic cops, preventing the immune response from veering off course.
- Anti‑inflammatory cytokines - such as IL‑10, they dial down inflammation once the danger is cleared.
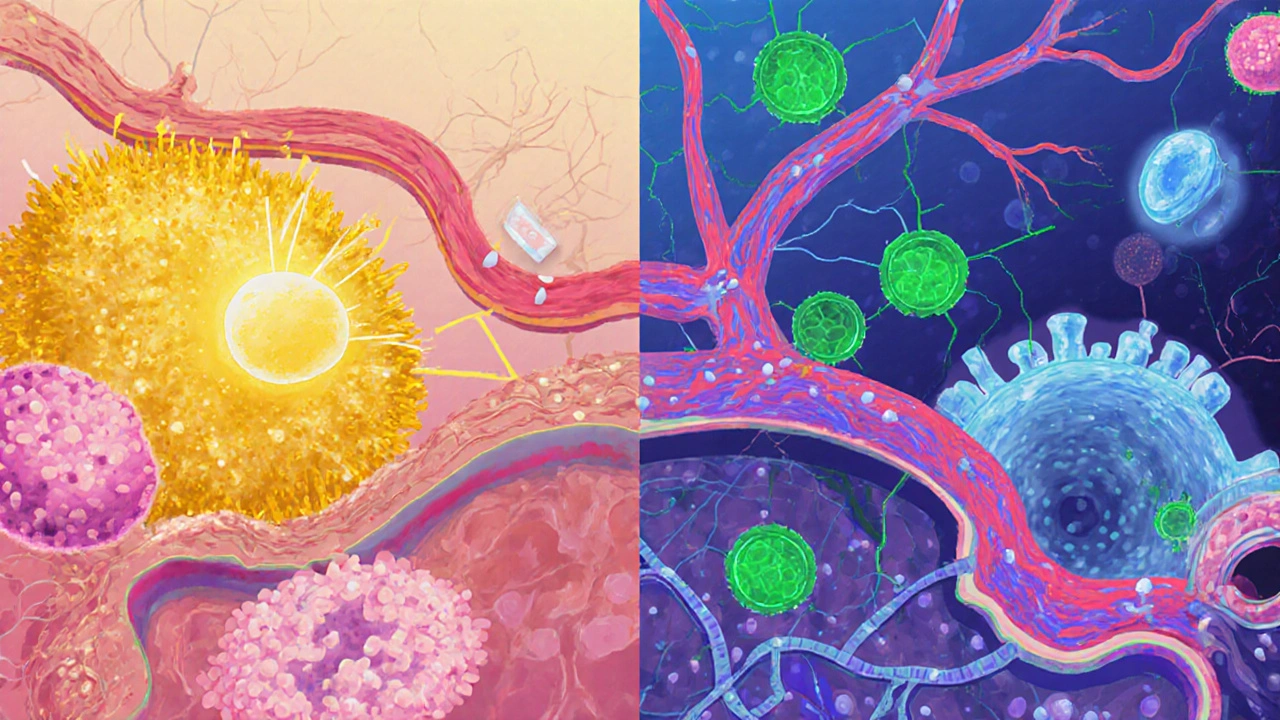
When these brakes fail, the immune system may start attacking harmless substances (allergies) or even the body’s own tissues (autoimmunity).
Allergic Disorders vs. Autoimmune Diseases - A Side‑by‑Side Look
| Feature | Allergic Disorders | Autoimmune Diseases |
|---|---|---|
| Typical Trigger | Harmless external agents (pollen, foods, dust mites) | Self‑antigens (thyroid, joints, pancreas) |
| Dominant Immune Pathway | IgE‑mediated, Th2 skewed | IgG/IgM‑mediated, Th1/Th17 skewed |
| Main Cytokines | IL‑4, IL‑5, IL‑13 | IFN‑γ, IL‑17, TNF‑α |
| Typical Symptoms | Itching, sneezing, wheezing, hives | Joint pain, organ dysfunction, chronic fatigue |
| Common Biomarkers | Elevated serum IgE, eosinophilia | Auto‑antibodies (ANA, RF, anti‑CCP) |
| Genetic Links | HLA‑DR, IL‑4Rα polymorphisms | HLA‑DRB1*04, PTPN22 variants |
| Environmental Triggers | Air pollution, diet, early‑life infections | Smoking, viral infections, gut dysbiosis |
Why Do the Two Conditions Overlap?
Research from the past decade points to three core mechanisms that blur the line between allergies and autoimmunity.
1. Chronic Inflammation as a Common Denominator
Both disease groups thrive on a state of low‑grade inflammation. Cytokines like TNF‑α and IL‑6 keep immune cells activated, creating a feedback loop that can convert a harmless IgE response into a self‑reactive attack.
2. Gut Microbiome Disruption
The gut microbiome is a community of trillions of bacteria that educates the immune system about what is safe and what is dangerous. Antibiotic overuse, high‑sugar diets, and lack of fiber reduce microbial diversity, lowering short‑chain fatty acids such as butyrate that normally promote Treg development. A 2023 Australian cohort study linked a low Bifidobacterium count with a 2.5‑fold rise in both asthma (an allergic disorder) and rheumatoid arthritis (an autoimmune disease).
3. Shared Genetic Susceptibility
Genome‑wide association studies (GWAS) have identified loci that predispose individuals to both conditions. For example, the IL‑4Rα gene variant rs3024656 increases IgE production and also heightens the risk of systemic lupus erythematosus. The overlap suggests that a single genetic “weak spot” can tip the immune balance in multiple directions.

Clinical Implications - What Doctors Need to Look For
If you’re already managing asthma, eczema, or hay fever, your clinician should be aware of the heightened risk for autoimmune disorders such as type 1 diabetes, thyroiditis, or multiple sclerosis.
Key steps for clinicians include:
- Screening for auto‑antibodies when patients present with persistent, unexplained fatigue or joint pain.
- Monitoring inflammatory markers like C‑reactive protein (CRP) and erythrocyte sedimentation rate (ESR) during allergy flare‑ups.
- Evaluating family history for both allergic and autoimmune conditions to gauge genetic risk.
Early detection can prevent irreversible organ damage and guide treatment choices.
Managing Overlapping Conditions - Integrated Strategies
Because the two disease families share pathways, treatment plans that target inflammation and immune regulation can benefit both.
Pharmacologic Options
- Biologics - Drugs like dupilumab (IL‑4/IL‑13 blocker) are approved for atopic dermatitis but also show promise in reducing inflammatory markers in ulcerative colitis, an autoimmune gut disease.
- Low‑dose naltrexone - Though off‑label, it modulates microglial activity and has been reported to improve symptoms in chronic urticaria and lupus.
- JAK inhibitors - Targeting cytokine signaling can simultaneously dampen allergic inflammation and autoimmune activity.
Lifestyle and Nutritional Interventions
Adopting habits that nurture the gut microbiome and lower systemic inflammation can have a double‑benefit.
- High‑fiber diet - Aim for 30g/day of mixed soluble and insoluble fiber (oats, legumes, berries) to feed beneficial bacteria.
- Fermented foods - Yogurt, kefir, kimchi introduce live cultures that can boost regulatory T‑cell numbers.
- Omega‑3 fatty acids - EPA/DHA from fish oil reduce IL‑6 and TNF‑α, easing both eczema and rheumatoid arthritis flares.
- Avoid smoking and excessive pollutants - Air quality directly influences both asthma severity and autoimmunity risk.
Monitoring Biomarkers
Regular labs can help track disease activity across both spectrums. Suggested panel every 6-12months:
- Serum total IgE and specific IgE (for allergic profile)
- Auto‑antibody panel (ANA, anti‑CCP, thyroid peroxidase)
- CRP and ESR (general inflammation)
- Stool analysis for dysbiosis (Bifidobacterium, Faecalibacterium)
Emerging Research - What’s on the Horizon?
Scientists are now testing a “dual‑modulation” approach: combining allergen‑specific immunotherapy (AIT) with immune checkpoint regulators to reset the immune system. Early phase‑II trials in Europe report that patients receiving AIT plus low‑dose PD‑1 inhibitors experienced reduced auto‑antibody titers alongside allergy symptom relief.
Another hot area is epigenetics. Methylation patterns on the FOXP3 gene, a master regulator of Tregs, differ in people who have both eczema and type 1 diabetes. Targeted dietary methyl donors (folate, B12) might one day be prescribed to correct these marks.
Lastly, personalized microbiome transplants are moving from theory to practice. A 2024 pilot study used donor stool enriched with Bifidobacterium longum to treat severe asthma patients; 40% also showed a drop in thyroid antibodies.
Take Action Now - A Simple Checklist
- Ask your doctor to screen for auto‑antibodies if you have persistent allergies.
- Include probiotic‑rich foods and 30g fiber daily.
- Consider anti‑inflammatory supplements like omega‑3s after consulting your clinician.
- Track symptom patterns - note if allergy flares precede joint pain or fatigue.
- Stay updated on clinical trials that target both pathways; registries often list eligibility criteria.
Frequently Asked Questions
Can having allergies cause an autoimmune disease?
Allergies don’t directly cause autoimmunity, but they share risk factors like chronic inflammation and genetic variants. Having one condition raises the odds of developing the other, especially if the immune system stays chronically activated.
Are there tests that check for both allergies and autoimmunity?
A comprehensive panel can include total IgE, specific IgE, and a standard auto‑antibody screen (ANA, RF, anti‑CCP). Adding CRP/ESR and stool microbiome analysis gives a fuller picture of underlying inflammation.
Do allergy shots help with autoimmune symptoms?
Allergen immunotherapy primarily recalibrates the allergic response. Some early studies suggest it can lower overall inflammatory markers, but it’s not a stand‑alone treatment for autoimmunity. It may be part of a combined strategy.
What lifestyle changes benefit both conditions?
Focus on gut health (high‑fiber, fermented foods), reduce exposure to pollutants, quit smoking, and include omega‑3 rich foods. Regular moderate exercise also lowers systemic cytokines that drive both allergy and autoimmunity.
Is there a genetic test that predicts both allergy and autoimmunity risk?
Direct‑to‑consumer panels now include HLA‑DR and IL‑4R variants, which correlate with elevated risk for both disease groups. However, genetics is only part of the picture; environment and lifestyle heavily modify the actual outcome.

Bart Cheever
October 2, 2025 AT 19:17Cut the fluff; stick to the facts.
Maude Rosièere Laqueille
October 11, 2025 AT 21:20There’s solid evidence that gut microbiota diversity influences both allergic and autoimmune pathways. Incorporating fermented foods like kefir or sauerkraut can help modulate the immune response. Also, omega‑3 fatty acids have anti‑inflammatory properties that may reduce auto‑reactivity. Monitoring vitamin D levels is another practical step, as deficiency correlates with higher disease susceptibility.
Amanda Joseph
October 20, 2025 AT 23:24Oh great, another calculator that tells you you’re probably doomed.
Kevin Aniston
October 30, 2025 AT 00:27I totally agree with the points about diet and microbiome, and I’d add that stress management plays a crucial role too. Chronic stress can elevate cortisol, which disrupts gut barrier integrity and may trigger autoimmune flare‑ups. Simple practices like daily meditation, adequate sleep, and moderate exercise have been shown to lower systemic inflammation. It’s also worth noting that personalized nutrition, guided by a professional, can fine‑tune the approach for each individual. Keeping a symptom diary helps track triggers and progress over time. Overall, a holistic lifestyle plan often yields the best outcomes for both allergy and autoimmunity management.
kiran kumar
November 8, 2025 AT 02:30Honestly, these risk calculators are just hype; you cant predict disease with a few dropdowns
Brian Johnson
November 17, 2025 AT 04:34That’s a solid overview. It’s helpful to see the connections laid out in one place.
Jessica Haggard
November 26, 2025 AT 06:37Nice summary! It’s fascinating how allergy mechanisms can overlap with autoimmune processes across different cultures and environments.
Alan Clark
December 5, 2025 AT 08:40Totally! And as we learn more, we’ll probably find even more shared pathways that can be targeted.
Mark Anderson
December 14, 2025 AT 10:44Wow, the interplay between the immune system and gut health is like a symphony-each instrument affecting the other in amazing ways!
Shouvik Mukherjee
December 23, 2025 AT 12:47I echo that excitement; encouraging patients to explore diet, environment, and stress factors can truly empower them on their health journey.
Jesse Stubbs
January 1, 2026 AT 14:50This is the worst health advice ever.
Melissa H.
January 10, 2026 AT 16:54Seriously? That’s a bit harsh 😅 Let’s keep the conversation constructive.
Edmond Abdou
January 19, 2026 AT 18:57Everyone’s experience varies, so it’s important to tailor recommendations rather than apply a one‑size‑fits‑all model.
Sydnie Baker
January 28, 2026 AT 21:00The emerging field of immunometabolism provides a compelling framework for understanding the convergence of allergic and autoimmune phenomena.
The recent meta‑analyses have demonstrated that dysregulated cytokine profiles, particularly elevated IL‑4 and IL‑17, are common denominators across seemingly disparate conditions.
Moreover, epigenetic modifications induced by environmental exposures can persist across generations, influencing both atopic predisposition and autoimmunity.
The intestinal barrier’s integrity, mediated by tight junction proteins such as claudin‑1 and occludin, emerges as a pivotal checkpoint.
When permeability is compromised, microbial antigens translocate, inciting systemic immune activation.
This process can precipitate a Th2‑skewed response in allergic individuals while simultaneously fostering Th1/Th17 pathways in autoimmune patients.
Nutritional interventions that enrich short‑chain fatty acid production, especially butyrate, have been shown to reinforce barrier function.
Concurrently, vitamin D receptor signaling exerts immunomodulatory effects by tempering dendritic cell maturation.
Pharmacologically, biologics targeting IgE or IL‑5 illustrate the therapeutic cross‑talk possible between allergy and autoimmunity treatment paradigms.
Yet, clinicians must remain vigilant about off‑target effects, as immune suppression can unmask latent autoimmune tendencies.
Clinical trials integrating microbiome sequencing with longitudinal symptom tracking are beginning to elucidate patient‑specific signatures.
These data underscore the necessity of precision medicine approaches that transcend traditional disease boundaries.
In practice, a comprehensive assessment should incorporate family history, environmental exposures, and gut health metrics.
Patients benefit from interdisciplinary collaboration among allergists, rheumatologists, gastroenterologists, and nutritionists.
Ultimately, embracing the shared immunological underpinnings will enhance diagnostic accuracy and therapeutic efficacy.
Benjie Gillam
February 6, 2026 AT 23:04The concept of immune cross‑talk invites us to reconsider the dichotomy between self and non‑self, suggesting that the immune system operates on a spectrum rather than binary states. By integrating systems biology, we can model these interactions and predict emergent disease patterns. Such frameworks encourage a more holistic therapeutic mindset. It’s an exciting frontier for research and patient care.
Naresh Sehgal
February 16, 2026 AT 01:07Don’t let the numbers scare you-take charge, tweak your diet, move your body, and watch your health improve!
Poppy Johnston
February 25, 2026 AT 03:10Seeing the big picture helps; remember that lifestyle, genetics, and environment all play roles.