When your machine measures a part to 0.001 mm precision, but it’s off by 0.005 mm, you don’t just have a bad reading-you have a batch of defective products, a failed audit, and possibly a patient at risk. In manufacturing, especially in medical devices, pharmaceuticals, and aerospace, equipment calibration isn’t a checklist item. It’s the foundation of trust in every measurement your plant makes.
What Calibration Actually Does
Calibration isn’t just adjusting a dial. It’s a formal process that compares your instrument’s readings to a known standard-something traceable to the International System of Units (SI). Think of it like checking your watch against the atomic clock at NIST. If your digital scale says 100 grams, but the certified weight says 99.8, you document that difference and correct the scale’s output. This isn’t optional. ISO 13485:2016 requires it. FDA 21 CFR Part 820 demands it. And if you skip it, you’re one inspection away from a warning letter.The real danger isn’t the occasional drift-it’s not knowing when it happens. A 2023 analysis of FDA warning letters showed 37.2% cited poor calibration practices. That’s not a small number. That’s a pattern. And it’s preventable.
Calibration Requirements You Can’t Ignore
ISO 13485:2016, Clause 7.6, is clear: all measuring equipment must be calibrated at specified intervals or before use. The standard doesn’t say “every six months.” It says “based on risk.” That means you get to decide the schedule-but you have to prove it makes sense.Here’s what that looks like in practice:
- High-precision micrometers in aerospace? Calibrated every 3 months.
- A basic thermometer in a food packaging line? Once a year.
- A pH meter in a humid cleanroom? Monthly, even if the manual says six.
Traceability is non-negotiable. Your calibration standard must link back to SI units through an unbroken chain of certified references. No “we got it from the supplier” excuses. NIST-traceable is the baseline in the U.S. BIPM-traceable is required in the EU under MDR 2017/745. If you export, you need both.
And don’t forget the environment. Calibration done in a 30°C warehouse won’t hold up in a 20°C lab. ISO 10012 and NIST Handbook 44 recommend 20°C ±2°C and 40% RH ±10%. If your workshop hits 35°C in summer, your calibration is meaningless.
Validation: When Calibration Isn’t Enough
Calibration tells you your tool reads correctly. Validation tells you your whole system works as intended.Think of it this way: Calibration checks the scale. Validation checks if the scale, the operator, the software, the ambient conditions, and the procedure together produce accurate results every time.
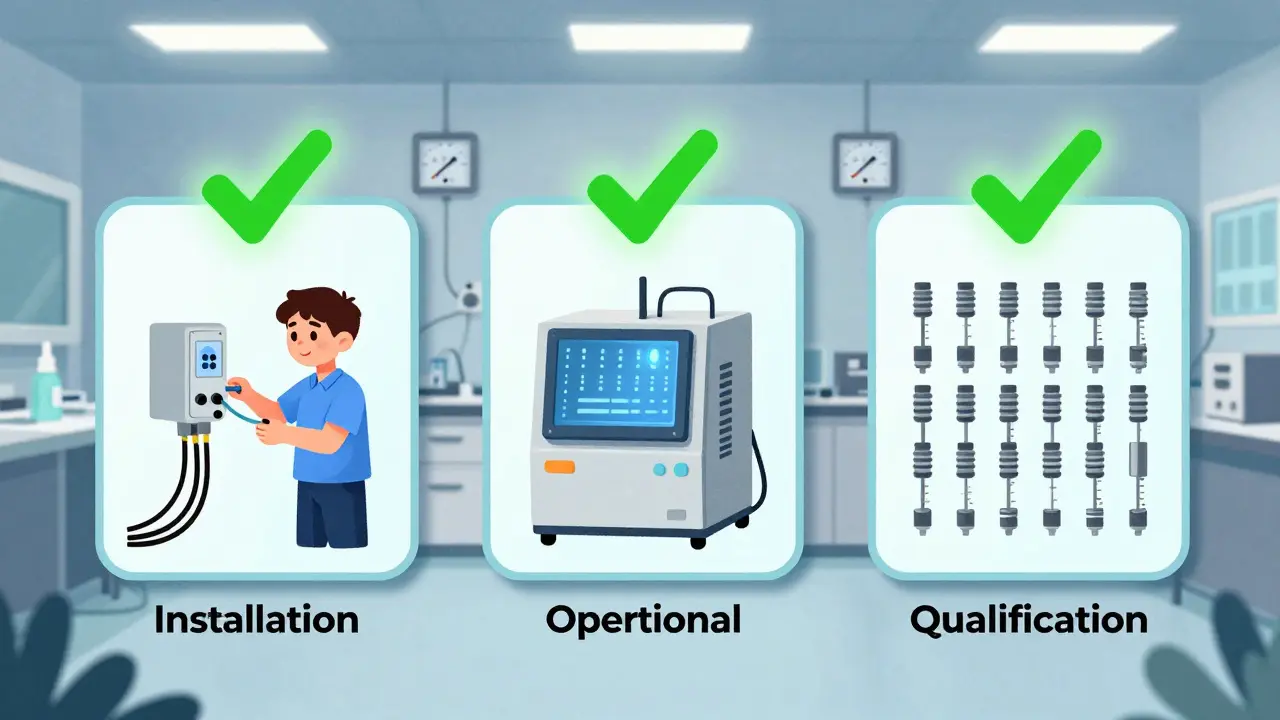
For medical devices, validation follows GAMP 5 guidelines with three phases:
- Installation Qualification (IQ): Did you install the machine right? Are the cables connected? Is the software version correct?
- Operational Qualification (OQ): Does it work under all expected conditions? Test it at max speed, min speed, high temp, low temp.
- Performance Qualification (PQ): Does it consistently produce good parts? Run 20 consecutive batches. Measure every critical dimension. Prove it’s repeatable.
Validation isn’t cheap. A single automated filling line can cost $250,000 to validate. But the cost of a recall? That’s millions. And the FDA doesn’t care if you’re a startup. If you make a Class II or III device, you’re expected to have done it.
Calibration vs. Validation: The Key Difference
People mix these up all the time. Here’s the simple breakdown:| Aspect | Calibration | Validation |
|---|---|---|
| Purpose | Ensure instrument accuracy against a standard | Prove the entire process works for its intended use |
| Focus | Equipment | System, procedure, environment |
| Frequency | Periodic (daily to yearly) | Once per installation, then revalidated after changes |
| Standard | ISO 13485, ISO 9001, CLIA | GAMP 5, FDA 21 CFR Part 11, EU MDR |
| Outcome | Calibration certificate | Validation report with acceptance criteria |
One doesn’t replace the other. You can have a perfectly calibrated machine that still produces bad results because the operator isn’t trained, the software has a bug, or the ambient humidity is too high. That’s why validation exists.
How to Set Your Calibration Intervals (Without Guessing)
Most companies start with the manufacturer’s recommendation. Then they realize it’s too frequent-or too lax.Successful organizations use Method 5 from SAE AS9100D:2016. It’s simple:
- Start with the manufacturer’s interval.
- Track your equipment’s performance over time. How often does it drift out of tolerance?
- Assess risk. What happens if it fails? Patient harm? Product recall? Regulatory fine?
- Adjust the interval based on data, not tradition.
One biomedical lab extended electronic scale calibration from quarterly to biannually after 18 months of data showed zero drift. Saved $18,500 a year. Another lab had to calibrate pH meters monthly because humidity in their room was too high. The manufacturer said six months. Reality said otherwise.
Don’t just follow the manual. Follow the data.
The Hidden Cost: Documentation and Time
The biggest complaint from small manufacturers? Paperwork.A 2024 FDA survey found small medical device companies spend 15.2 hours a week just managing calibration records. That’s nearly two full days. And if you’re using spreadsheets or paper logs, you’re already behind.
Companies using cloud-based calibration software like GageList or Trescal cut audit prep time by 63%. Automated reminders, digital certificates, and integrated ERP links make compliance possible without hiring a full-time quality admin.
But here’s the catch: 32.7% of negative reviews cite integration problems with old systems like SAP ECC 6.0. If you’re still on legacy software, budget for upgrades. The cost of a failed audit is higher than the cost of a new system.
What’s Changing in 2025 and Beyond
Regulations aren’t standing still.ISO 13485:2016 Amendment 1, released in March 2024, now requires calibration of AI and machine learning systems. If your quality control uses an algorithm to predict defects, you need to validate that algorithm’s inputs and outputs continuously. Drift in the model? That’s a calibration issue now.
The FDA’s 2024 Calibration Modernization Initiative requires all Class II and III device manufacturers to switch to electronic records by December 31, 2026. That’s not a suggestion. That’s a deadline. Paper logs will no longer be acceptable.
NIST is also working on quantum-based calibration standards that could make electrical measurements 100 times more accurate by 2030. That’s not science fiction-it’s happening in labs right now. The tools you buy today might need to be replaced sooner than you think.

Who’s at Risk? The Real-World Traps
Here’s what goes wrong-and how to avoid it:- Ignoring environmental conditions: 57.8% of out-of-tolerance events happen when temperature or humidity changes. Install monitoring sensors. Log data. Don’t assume.
- Using untraceable standards: Don’t buy a “calibration weight” off Amazon. Get one with a certificate from an accredited lab.
- Not training staff: If your technician doesn’t know how to handle a reference standard, calibration is meaningless. Train them. Document it.
- Skipping revalidation after changes: Changed the software? Moved the machine? Replaced a sensor? That triggers a full revalidation. Not a quick check.
Dr. James Westad, an ASQ Fellow, puts it bluntly: “Organizations wasting resources on monthly calibrations of stable equipment while neglecting environmental monitoring are violating ISO 13485’s risk-based philosophy.”
That’s the future: smart, data-driven, condition-based calibration-not calendar-based.
Final Checklist for Compliance
Before your next audit, ask yourself:- Do I have a full inventory of all measuring equipment, each with a unique ID?
- Is every calibration traceable to SI units with documented uncertainty?
- Are calibration intervals based on data, not guesswork?
- Are environmental conditions monitored and logged during calibration?
- Are calibration records retained for at least the product lifecycle plus 2 years?
- Have I validated all critical production systems using IQ/OQ/PQ?
- Are all calibration and validation records electronic and searchable?
- Have I trained staff on proper handling and documentation?
If you can answer yes to all of these, you’re not just compliant. You’re building a quality culture.
What happens if I don’t calibrate my equipment?
If you skip calibration, you risk producing defective products, failing regulatory audits, and facing FDA warning letters or product recalls. In medical device manufacturing, this can directly impact patient safety. A 2023 FDA analysis found 37.2% of warning letters cited inadequate calibration. The financial and reputational damage can be severe.
How often should I calibrate my equipment?
There’s no universal rule. Calibration frequency depends on the equipment, its use, and environmental conditions. High-precision tools like micrometers may need quarterly calibration, while basic thermometers might only need annual checks. Use historical performance data and risk assessment to determine intervals-not just manufacturer recommendations.
Is calibration the same as validation?
No. Calibration ensures a measuring device reads accurately against a known standard. Validation confirms that an entire system-equipment, software, procedures, and environment-consistently produces the intended results. You need both. A perfectly calibrated machine can still fail validation if the process around it is flawed.
Can I calibrate my own equipment?
Yes, if you have the right standards, trained personnel, and documented procedures. Many larger manufacturers have in-house calibration labs. But you must prove traceability to SI units and maintain full documentation. For most small companies, using an accredited third-party lab is more practical and less risky.
What’s the biggest mistake companies make with calibration?
The biggest mistake is treating calibration as a calendar task instead of a risk-based process. Many companies calibrate everything on the same schedule, regardless of usage or environment. This wastes money and misses real risks. The smart approach uses data to adjust intervals-calibrate more often if equipment drifts, less if it stays stable.
Do I need certification for my calibration lab?
If you’re doing calibration in-house, you don’t need external certification-but your process must meet ISO 17025 standards for competence. If you’re outsourcing, always use an ISO/IEC 17025 accredited lab. Their certificates carry legal weight during audits. Non-accredited labs may give you a paper, but regulators won’t accept it.
Next Steps: Where to Start
If you’re starting from scratch:- Inventory every measuring device in your facility. Give each one a unique ID.
- Classify them by risk: critical (directly affects product safety), important (affects quality), and basic (general use).
- For critical devices, set up calibration intervals using Method 5 (manufacturer + data + risk).
- Invest in digital calibration software. It’s not optional anymore.
- Train your team. Calibration isn’t just the job of one person-it’s everyone’s responsibility.
Quality isn’t something you add on. It’s built into every step. And if you’re not calibrating properly, you’re not building quality-you’re building risk.

Monte Pareek
December 17, 2025 AT 18:34Man I've seen so many shops skip calibration because it's "too expensive" or "the machine has always been good" until the FDA shows up with a warning letter and they're scrambling to explain why their pH meter was calibrated in a warehouse that hits 95°F in July
It's not about the tool-it's about the data. Track drift. Adjust intervals. Stop guessing. That's how you build real quality, not just paperwork.
Allison Pannabekcer
December 19, 2025 AT 10:18This is such a clear breakdown. I work in pharma and I see people confuse calibration and validation all the time. Calibration is like checking your ruler. Validation is making sure the whole damn factory can build the same thing every single time, even when the AC breaks or someone forgets to wear gloves.
Both matter. Neither is optional. Thanks for laying it out like this.
Sarah McQuillan
December 21, 2025 AT 05:02Let me tell you something about NIST traceability. In my opinion, it's all a government scam to sell more expensive equipment. I calibrated my micrometer with a weight I bought off eBay and it worked fine for three years. No one got hurt. No one even noticed. Why do we need to pay $500 for a certificate when common sense works better?
Also, quantum standards? That's just sci-fi nonsense. We're still using calipers. Don't let them scare you into buying new gear.
Aboobakar Muhammedali
December 22, 2025 AT 11:43I come from India where we don't have access to fancy labs or cloud software but we still make medical devices that save lives
Our team uses a simple system: we write down every calibration on a whiteboard, take a photo, and email it to the QA lead. No fancy software. Just discipline. We’ve passed three audits like this
It’s not about the tool-it’s about the person holding it. If you care, you’ll do it right. Even with paper.
anthony funes gomez
December 24, 2025 AT 05:30Calibration is not merely a procedural artifact-it is the epistemological anchor of metrological integrity within the manufacturing episteme. Without traceability to SI units, the ontological status of measurement becomes contingent, unstable, and ultimately epistemologically invalid. The validation process, then, functions as a hermeneutic closure mechanism-ensuring that the apparatus, operator, environment, and algorithmic output cohere into a semiotically consistent operational regime.
And yes, if your calibration interval is determined by calendar time rather than stochastic drift analysis, you are not managing risk-you are performing performative compliance.
Laura Hamill
December 24, 2025 AT 18:22They’re coming for our paper logs. I know it. I’ve seen the emails. The FDA is working with Big Tech to replace our handwritten logs with AI that tracks every screw we tighten. Next they’ll implant chips in our micrometers and monitor our breathing while we calibrate.
They say it’s for safety. I say it’s control. I’ve been using the same pen and notebook since 2010. It’s never failed me. You think they care about patient safety? Nah. They care about data. And data means power.
Don’t let them take your pen. 🤫👁️
William Storrs
December 25, 2025 AT 15:56Great post. Seriously. I’ve been in this game 20 years and I still see shops treating calibration like a quarterly chore.
Here’s the thing: you don’t need a $100k system to get started. Just pick one critical piece of equipment. Track its drift for 6 months. Adjust the interval. Document it. Then do it again with the next one.
Small wins build culture. You don’t need perfection-you need progress. Keep going.
James Stearns
December 26, 2025 AT 22:49While I appreciate the general sentiment conveyed herein, I must register a formal objection to the casual tone and lack of institutional gravitas in the presentation of these critical regulatory imperatives.
It is not sufficient to merely "track drift"-one must establish a documented, auditable, and fully validated metrological control system in accordance with ISO 10012:2003, NIST Handbook 150-2, and the applicable provisions of 21 CFR Part 820.30. To suggest otherwise is to engage in a dangerous dilution of quality assurance principles.
Furthermore, the use of emoticons in public discourse is an affront to professional standards.
Marsha Jentzsch
December 28, 2025 AT 09:04Wait, so you're telling me if I don't calibrate my scale, someone might DIE??
That's terrifying. I just thought it was about getting the right number of grams for my protein powder. What if my baby's medicine was made on that machine??
My hands are shaking. I need to go check my lab right now. I didn't even know this was a thing. I'm so sorry.
Someone please tell me I'm not going to jail.
Janelle Moore
December 29, 2025 AT 06:56Everyone’s talking about calibration like it’s some big secret. Here’s the truth: 90% of the time, the problem isn’t the machine. It’s the guy who dropped it. Or the one who didn’t let it warm up. Or the one who used a dirty weight.
Train your people. Stop blaming the tool. And stop buying expensive software because you’re too lazy to write things down.
My shop uses Excel. We’ve never failed an audit. Because we care. Not because we paid for a subscription.
Henry Marcus
December 29, 2025 AT 23:57Quantum calibration by 2030? LOL. You think the FDA cares about that? They’re still chasing paper logs. Meanwhile, the real revolution is happening in China-they’re using AI to predict calibration drift before it happens. No sensors. No standards. Just algorithms trained on 10 million measurements.
They’re not waiting for NIST. They’re building their own reality.
And guess what? Their devices are cheaper, better, and FDA-approved. We’re not just behind. We’re being left behind by a system that still thinks a ruler is a tool.
Wake up.